The ERN EURO-NMD Registry
The Euro-NMD Registry is a web-based platform designed in RedCap and hosted by the Universitäts Klinikum Freiburg. It intends to collect data from all neuromuscular patients seen by the 82 HCPs participating in the Euro-NMD network.
In parallel, existing disease-specific registries can participate in the Registry Hub. This FAIR-based infrastructure allows federated queries to be run across all the different datasets and returns aggregate results for all the registries connected through the Hub.
The EURO-NMD Registry enables uniform data capture and sharing across all network members through a regulated Data Access Policy. It is designed to improve the prevention, diagnosis and treatment of Neuromuscular Disorders.
Aims of the ERN EURO-NMD Registry
The Registry’s primary aim is to support our patients’ care and guarantee the delivery of the highest standards of diagnosis and treatment across Europe while providing follow-up and improved management for undiagnosed patients.
At the same time, the Registry enables research opportunities for clinical trials, translational research and epidemiological studies, increasing patients’ access to research and knowledge generation.
- Monitoring care delivery by Key Performance Indicators based on clinical and patient outcome measures.
- Pseudo-anonymised data collection for all the patients seen within the network by each of the 82 healthcare providers that compose EURO-NMD
- The possibility of aggregating existing data sources for neuromuscular diseases to our data collection, with or without data transfer per existing regulations, namely GDPR. The Registry Hub guarantees the data analysis, without data transfer, from different data sources, connected via data FAIR points, subject to regulated access as detailed in the Data Access Policy
- Provide a user-friendly platform simplifying data entry tasks with time-saving customisation of the workflow by branching logic
- Demographic data collection helps build a clearer epidemiological picture of neuromuscular disorders in Europe.
- Identification of patient cohorts for clinical research, namely participation in clinical trials.
- Natural history with deep phenotyping and continuous longitudinal real-world data collection for specific neuromuscular disease subsets.
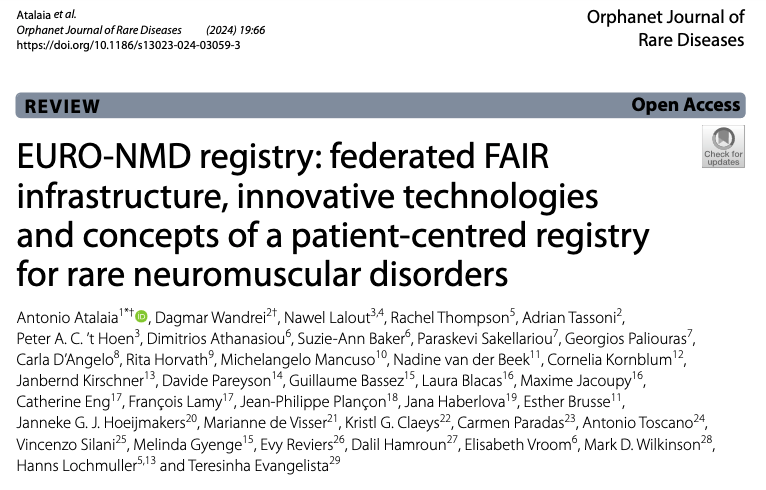
Sets of data items in the ERN EURO-NMD Registry
The registry has three data levels:
- EU-CDEs are the set of Common Data Elements chosen by the Joint Research Centre and the European Platform on Rare Disease Registration for collection by all the European Reference Networks. These CDEs need to be collected once per year for each patient seen by the EURO-NMD’s healthcare providers.
- NMD-CDEs: This dataset contains all the cross-neuromuscular data elements useful for any neuromuscular condition. This dataset is recommended but not mandatory until 2026.
- DS-DEs: disease-specific data elements are fields specific for each condition or group of conditions, as defined by the 5 working groups.
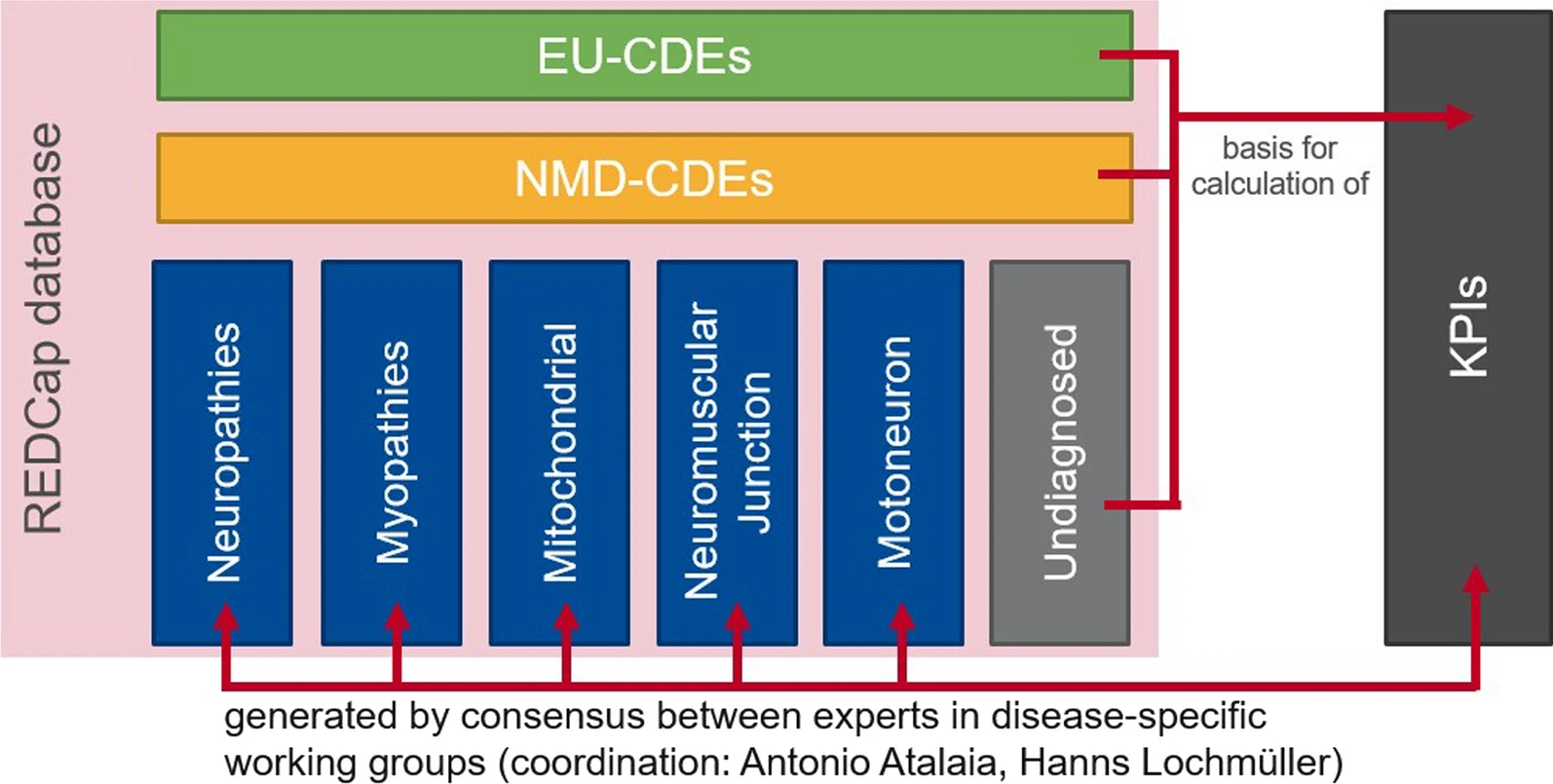
Why EURO-NMD Registry Hub?
- The EURO-NMD registry is connected to what is called ‘EURO-NMD Registry Hub’ through an interoperability layer
- The Hub provides an entry point to other neuromuscular registries that follow the FAIR data stewardship principles and enable GDPR-compliant information exchange
- Four national or disease-specific patient registries are interoperable with the EURO-NMD Registry, allowing for federated analysis across these different resources.
- Collectively, the Registry Hub brings together data that are currently siloed andfragmented to improve healthcare and advance research for neuromuscular diseases.
- The EURO-NMD Registry Hub is designed as a clinician-patient partnership.
Registry Governance
| Coordinator | Teresinha Evangelista | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Clinical Advisor | António Atalaia | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Project Manager | Carla D’Angelo | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Co-Coordinator | Janbernd Kirschner | Medical Center – University of Freiburg, Germany |
| Data Manager | Petar Horki | Medical Center – University of Freiburg, Germany |
| IT Helpdesk | Petar Horki | Medical Center – University of Freiburg, Germany |
| IT Lead | Adrian Tassoni | Medical Center – University of Freiburg, Germany |
| Interoperability and FAIRification Lead | Peter-Bram t’Hoen | RadboudUMC, Netherlands |
| Interoperability and FAIRification, FAIR Data Steward | Nawel Lalout | RadboudUMC, Netherlands |
The registry is governed by a Registry Steering Committee composed by the EURO-NMD Coordinator and Members of the EURO-NMD Registry Consortium. The Registry Steering Committee works closely with the EURO-NMD Executive Committee where each thematic working group of the network is represented.