Orphanet has developed and maintains the Orphanet nomenclature of rare diseases, that is cross-referenced with other international terminologies and reference databases (including OMIM, ICD-10, SNOMED-CT, MedDRA, UMLS, MeSH, and GARD) in order to enable interoperability between different information systems. The Orphanet classification is organised according to three hierarchical levels: Group of disorders, Disorder, and Subtype of a disorder, that determine the level of precision of each diagnosis included in the nomenclature. This classification level is indicated on the respective Orphanet website page of each clinical entity.
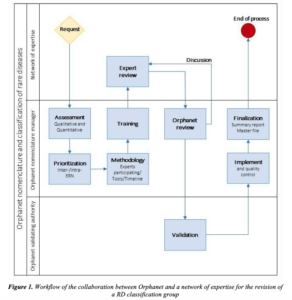
The European Commission has recommended the adoption of Orpha codes for designating rare diseases covered by the 24 European Reference Networks for Rare Diseases (ERNs). As knowledge evolves, there is need to create new entries and modify existing ones. Orphanet has established a procedure to support ERNs in this process: “Procedural document: Collaboration with networks of expertise for the revision of the Orphanet nomenclature and classification of rare diseases”.
The scheme that summarizes the process is depicted in the below figure, copied from the above cited document: