30 Nov 2017
International-DMD (IDMD): a PTC Therapeutics-supported diagnostic project to identify Dystrophin mutations by NGS technologies in patients throughout European countries

Authors:
Fernanda Fortunato (frtfnn@unife.it), Fortunato Fernanda (1), Selvatici Rita (1), Rossi Rachele (1), Mauro Antonio (1), Armaroli Annarita (1), Neri Marcella (1), Trabanelli Cecilia (1), Fabris Marina (1), Rimessi Paola (1), Fini Sergio (1), Gualandi Francesca (1), Ferlini Alessandra (1)
1)Unit of Medical Genetics, Department of Medical Sciences, University of Ferrara, Ferrara, Italy
Duchenne Muscular Dystrophy (DMD) is an X-linked inherited neuromuscolar disorder due to mutations in the dystrophin gene. First signs and symptoms of DMD include delayed milestones such as walking and talking and enlarged calves.
Given that the progressive muscle wasting is irreversible, it is important that genetic diagnosis be defined as early as possible to consider all potentially therapeutic options early in the disease course.
PTC Therapeutics International Ltd. and the University of Ferrara, Italy, have established a collaboration focused on identifying patients affected by rare genetic disorders. Genetic testing is available to patients throughout European countries, potentially expanding to other regions, with an initial focus on DMD.
Diagnostic settings include MLPA (MRC Holland) and NGS dystrophin gene sequencing (Multiplicom).
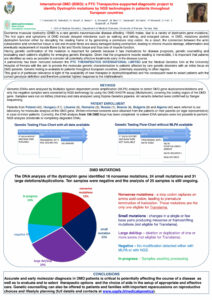
Currently DNA from 116 DMD boys was collected and 6 females were tested too.
Patients were from: Poland (42), Hungary (17), Lituania (6), Romania (3), Russia (1), Bosnia (4), Bulgaria (9); moreover, 40 samples from Algeria were analyzed.
Among the samples analyzed, 16 nonsense mutations, 24 small mutations, 31 deletions/duplications were detected; 10 patients tested negative and the analysis is still ongoing in 25 samples.
For 16 samples, an additional DNA amount was required as DNA sent was not adequate to genetic analysis.
This collaborative project demonstrates PTC’s commitment to expanding awareness of the importance of genetic diagnosis for patients with DMD.
Accurate and early molecular diagnosis in DMD patients is critical to potentially affecting the course of the disease as well as to evaluate and select therapeutic options and the choice of aids in the setup of appropriate and effective care.
Genetic counselling can also be offered to patients and families with important repercussions on reproductive choices and lifestyle planning (full details and contacts at www.ospfe.it/medicalgenetics).