A recognised challenge for rare diseases is the heterogeneity of legacy data sets and the multiplicity of existing registries. EURO- NMD health care providers and patient organizations are currently active in more than 120, mostly disease specific and patient run registries.
While the existing registries are collecting important information, none of them is used by all EURO-NMD centres and there is no unified NMD or NMD Disease Specific Registry in EU.
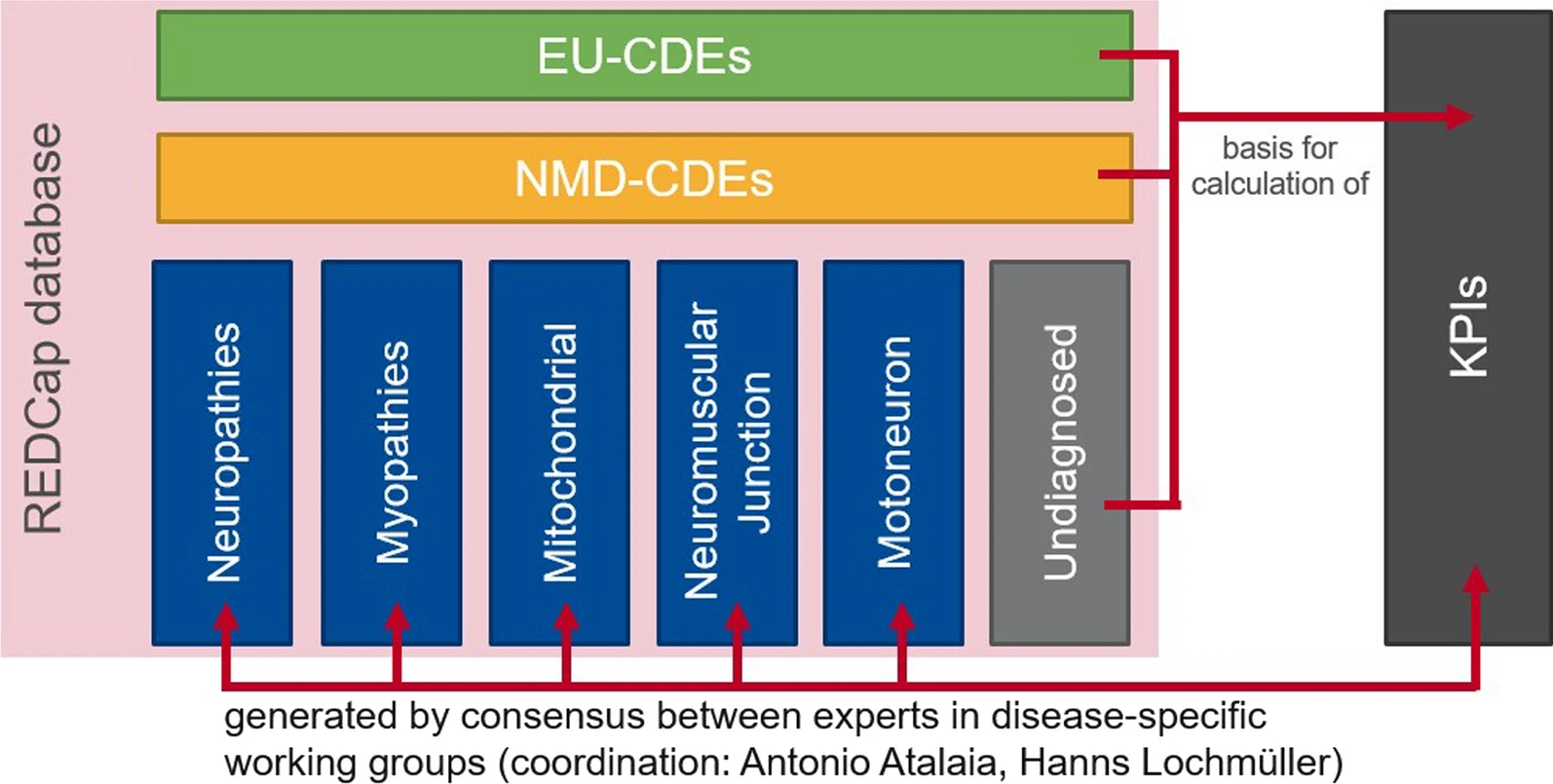
The Euro-NMD Registry is a web-based platform designed in RedCap and hosted by the Universitäts Klinikum Freiburg. It intends to collect data from all neuromuscular patients seen by the 82 HCPs participating in the Euro-NMD network.
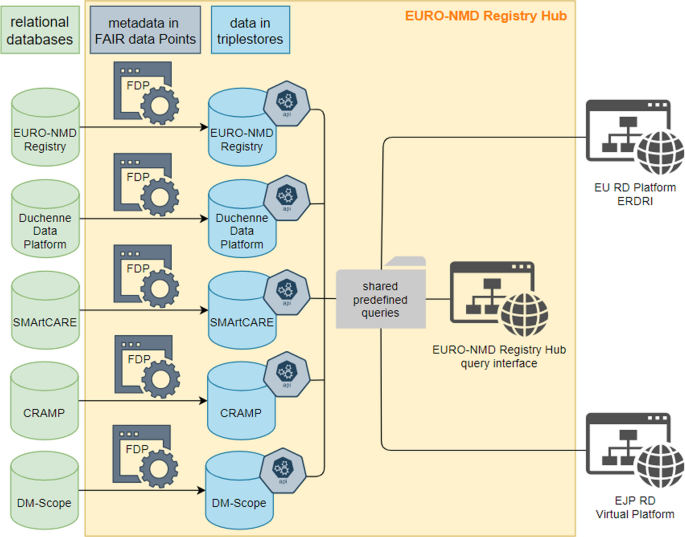
In parallel, existing disease-specific registries can participate in the Registry Hub. This FAIR-based infrastructure allows federated queries to be run across all the different datasets and return aggregate results for all the registries connected through the Hub.
The EURO-NMD Registry enables uniform data capture and sharing across all network members through regulated Data Access Policy. It is designed to improve the prevention, diagnosis and treatment of Neuromuscular Disorders.
The Registry’s primary aim is to support our patients’ care and guarantee the delivery of the highest standards of diagnosis and treatment across Europe while providing follow-up and improved management for undiagnosed patients.
At the same time, the Registry enables research opportunities for clinical trials, translational research and epidemiological studies, increasing patients’ access to research and knowledge generation.
The registry has three data levels:
| Coordinator | Teresinha Evangelista | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Clinical Advisor | António Atalaia | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Project Manager | Carla D’Angelo | APHP Hôpital Pitié Salpêtrière, Paris, France |
| Co-Coordinator | Janbernd Kirschner | Medical Center – University of Freiburg, Germany |
| Data Manager | Dagmar Wandrei | Medical Center – University of Freiburg, Germany |
| IT Helpdesk | Dagmar Wandrei | Medical Center – University of Freiburg, Germany |
| IT Lead | Adrian Tassoni | Medical Center – University of Freiburg, Germany |
| Interoperability and FAIRification Lead | Peter-Bram t’Hoen | RadboudUMC, Netherlands |
| Interoperability and FAIRification, FAIR Data Steward | Nawel Lalout | RadboudUMC, Netherlands |
The registry is governed by a Registry Steering Committee composed by the EURO-NMD Coordinator and Members of the EURO-NMD Registry Consortium. The Registry Steering Committee works closely with the EURO-NMD Executive Committee where each thematic working group of the network is represented.
Learn how to use the essential features of the Registry: click to read in a new tab
Standard Operating Procedures of the Registry Steering Committee
Standard Operating Procedures of the Data Access Committee
Informed Consent Form in English
EURO-NMD Registry Codebook by EURO-NMD Registry shared under the terms of a Creative Commons CC-BY-NC-SA license.
EURONMD Annual Meeting 2024 Registry Slides
Find here the Data Access Policy of the EURO-NMD Registry: click to open in a new tab
Form to request access to data: click here to open in a new tab (UNDER CONSTRUCTION)
Data Acess Committe Feedback form: click here to open in a new tab (UNDER CONSTRUCTION)
For other queries use our helpdesk email address please: registryhelpdesk.euronmd@outlook.com
Click here to open Registry Onboarding information in a new tab




