
All citizens of the European Union have the right to cross-border healthcare.
The European Commision has published a study supporting the evaluation of Directive 2011/24/EU to ensure patients’ rights in the EU in cross-border healthcare, a Commission Report on the operation of the directive, and data on patient mobility under the directive from 2018-2020.
Patients travelling to another EU country for medical care will enjoy equal treatment with the citizens of the country in which they are treated. If they are entitled to that healthcare at home, then they will be reimbursed by their home country. Their reimbursement will be up to the cost of that treatment at home. In some cases, they may need to seek authorisation before travelling for treatment, in particular if the treatment requires an overnight stay at an hospital or highly specialised and cost-intensive healthcare.
EU citizens have the right to access healthcare in any EU country and to be reimbursed for care abroad by their home country.
Directive 2011/24/EU on patients’ rights in cross-border healthcare sets out the conditions under which a patient may travel to another EU member state to receive medical care and reimbursement. It covers healthcare costs, as well as the prescription and delivery of medications and medical devices.
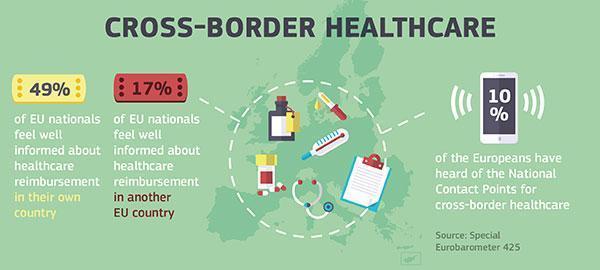
With health policies and systems increasingly interconnected, the Directive makes it easier to access:
The provisions strike the right balance between maintaining the sustainability of health systems, while protecting patients’ right to seek treatment outside their home country.
The Directive :
To know before you go
For more information on cross-border healthcare, you should contact the national contact point in your country. Find out where your national contact point is located HERE.
The Directive 2011/24/EU on patients’ rights in cross-border healthcare clarifies the rules regarding medical care in another EU country and the conditions to be met to qualify for a refund. Watch the video of the European Commission.




