|
| |
Translational Summer School Special 2018!!
|
|
|
|
| |
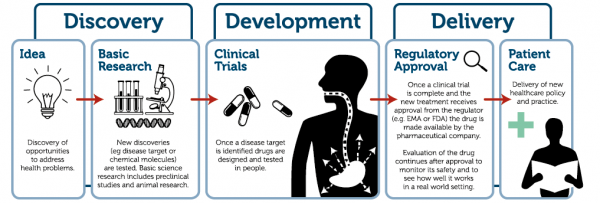
Our translational summer school is fast approaching and we have a few places still available for those who are interested in attending. This month's newsletter features a comprehensive overview of the programme, highlighting the key stakeholders attendees as well as the objectives of each session.
The course, which is run in association with TREAT-NMD, costs €500 will be held 2-6 July in Newcastle-upon-Tyne, UK.
Attendees will benefit from a comprehensive grounding in the translational research pathway from bench to bedside. The final programme is highlighted below in this special newsletter.
Although this course focuses on translational research in the context of neuromuscular diseases, the processes, principles and concepts that underpin this summer school are applicable to many different disease areas. We would warmly welcome attendees from these different disease areas as their experiences, understanding and input would only go further to enhance the learning environment for all concerned and we would encourage them to apply.
|
|
|
|
| |
"Our comprehensive course will be especially beneficial to those people working in clinical trials."
|
|
|
|
| |
Session 1 - The Translational Lifecycle
|
|
|
|
| |
Overview of bench to
bedside
Annemieke Aartsma-Rus
Leiden University Medical Center
Netherlands
Objective: to outline the different steps of therapy development from idea, to proof of concept studies in model systems, to preclinical optimization studies, clinical trials, drug approval and post marketing surveillance studies
|
Tools of the trade for preclinical Research
Volker Straub &
Annemieke Aartsma-Rus
Institute of Genetic Medicine
Newcastle University, UK & Leiden University Medical Center, Netherlands
Objective: outline different models in preclinical research, their opportunities and limitations, and the need for standardized tests and the TREAT-NMD advisory committee for therapeutics (TACT)
|
|
|
|
|
|
| |
How the regulatory systems works
Violeta Stoyanova-Beninska
Medicines Evaluation Board
Netherlands
Objective: explain the centralized system, how it is organized and how regulators decide whether drugs are eligible for marketing authorization, outline patient involvement in committees – focus on rare diseases
|
Mock TACT review
session
Annemieke Aartsma-Rus, Volker Straub, & Cathy Turner
Leiden University Medical Center, Netherlands & Institute of Genetic Medicine, Newcastle University, UK
Objective: learning to have a critical look at preclinical research. In this mock session participants will have been provided with a fictitious TACT application from a company planning a clinical trial in the NMD space. Participants will be split into groups of 8-10 participants to discuss the strengths and limitations and outstanding questions of the application.
|
|
|
|
|
|
| |
Session 2 - Clinical Trials
|
|
|
|
| |
Introduction to clinical
trials
Michela Guglieri
Institute of Genetic Medicine
Newcastle University, UK
Objective: introduce how and why clinical trials are conducted, the objective of different trial phases, trial design, primary endpoints, secondary endpoints, clinical significance, ethical concerns and informed consent
|
Ethical discussion &
role play
Silvere van der Maarel, Becca Leary
& Teresinha Evangelista
Leiden University Medical Center, Netherlands & Institute of Genetic Medicine, Newcastle University, UK
Participants will be provided with a scenario for a clinical trial plan for a drug to be tested in children. Different roles will be given to different participants.
Objective: gain insight in ethical discussions related to clinical trials and the perspectives of different stakeholders
|
|
|
|
|
|
| |
Outcome
measures
Anna Mayhew
Institute of Genetic Medicine
Newcastle University, UK
Objective: overview of the different types of functional outcome measures used in clinical trials for neuromuscular, their opportunities and limitations, need for standardization and training, TREAT-NMD efforts in their area, natural history studies and their usefulness in trial design
|
Showcase on outcome measure development & labelling & marketing
Annemieke Aartsma-Rus
Leiden University Medical Center, Netherlands
Objective: to illustrate the consequences of using outcome measures limited to certain disease stages with regards to extrapolation and limited indications
|
|
|
|
|
|
| |
Showcase on outcome
measure development
Anna Mayhew
Institute of Genetic Medicine
Newcastle University, UK
Objective: to outline the steps and stakeholders involved in developing and validating functional outcome measures that assess what patient find important using upper limb function outcome measure development for DMD and SMA as showcases
|
TREAT-NMD tools to facilitate clinical trials
Michela Guglieri & Becca Leary
Institute of Genetic Medicine
Newcastle University, UK
Objective: to gain insight in available tools and services for planning and conducting clinical trials
|
|
|
|
|
|
| |
Biomarkers
Pietro Spitali & Andreas Roos
Leden University Medical Center, Netherlands & Institute of Genetic Medicine, Newcastle University, UK
Objective: to explain why types of biomarkers exist and how they can be used in trial planning and as outcome measures, the regulatory perspective on biomarkers, highlighting ongoing networking efforts (ENMC workshops)
|
Showcase: validation of MRI as a biomarker in clinical trials
Volker Straub
Institute of Genetic Medicine,
Newcastle University, UK
Objective: introduce MRI as an outcome measure in trials, ongoing efforts to validate this biomarker for assessment of muscle quality in neuromuscular diseases
|
|
|
|
|
|
| |
Showcase:
PROM development
Nathalie Goemans
University Hospitals Leuven, Belgium
DVC St Jozef Antwerp, Belgium
Objective: explain what happens after a drug is approved, marketing, postmarketing studies, reimbursement and challenges around drug access
|
After EMA approval: lessons learned, challenges
Ria Broekgaarden
VSN; Dutch Patient Society of Neuromuscular Diseases
Interactive workshop with Ria Broekgaarden (VSN Netherlands, PAB member EURO-NMD) to discuss challenges around drug access, start stop criteria and drug migration
|
|
|
|
|
|
| |
Role of patient organisations in research: examples, achievements from start up to registries
Ria Broekgaarden
VSN; Dutch Patient Society of Neuromuscular Diseases
Objective: to learn how patients are equal partners in drug development and can be instrumental in the processes involved in drug development
|
Clinical trial practicality forum
Teresinha Evangelista
Institute of Genetic Medicine,
Newcastle University, UK
Participants: trial nurse, clinician involved in clinical trials, patients involved in clinical trial, patient representatives
In this panel session participants will briefly introduce themselves and their personal experience with being involved in a clinical trial as a health care professional or a patient.
What is it like? What is challenging? What is burdensome? Where things going as expected? This is followed by an interactive panel discussion where participants can ask questions and contribute to the discussion
|
|
|
|
|
|
| |
Patient communication workshop:
Importance of patient communication |
|
|
|
| |
Communication workshop by behavioural scientists
Objective: During this workshop participants will learn about unconscious processes, association and framing, which influence how what is communicated is received by the recipient.
|
|
|
|
| |
Participant presentations |
|
|
|
| |
Communication workshop by behavioural scientists
We ask each group of 3-4 participants to prepare a 10-15 minute talk - Who they are and what they expected from the summer school
- The things they learnt
- How this will influence their daily work?
- What we should keep in future summer schools?
- What we should drop/improve?
What they were missing?
|
|
|
|